Abstract
Background/Introduction: Zanubrutinib is a potent, irreversible Bruton tyrosine kinase (BTK) inhibitor designed to maximize BTK occupancy and minimize off-target kinase inhibition. Zanubrutinib is approved globally for the treatment of B-cell malignancies in adults. Here, we present the efficacy and safety of zanubrutinib assessed in patients with mature B-cell malignancies enrolled in the ongoing, multicenter, open-label phase 1/2 study in Japan (BGB-3111-111; NCT04172246).
Methods: In part 1, safety and tolerability of zanubrutinib were evaluated in 6 enrolled patients with mature B-cell malignancies who received zanubrutinib 160 mg orally twice daily. In part 2, the safety and efficacy of zanubrutinib were evaluated in 47 enrolled patients with relapsed/refractory (RR) mantle cell lymphoma (MCL), chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), and Waldenström macroglobulinemia (WM). Responses were assessed by investigators based on the Lugano Classification for MCL and SLL, 2018 International Workshop on CLL (iwCLL) guidelines with modification for treatment-related lymphocytosis for CLL, and WM response criteria updated at the 6th International Workshop on WM.
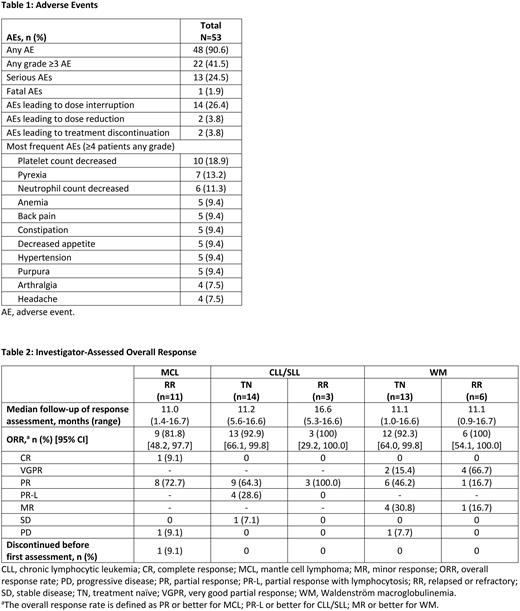
Results: As of May 10, 2022, 53 patients have been enrolled (12 RR MCL; 17 CLL/SLL; 21 WM; 2 follicular lymphoma; 1 marginal zone lymphoma). Fourteen (26.4%) patients discontinued treatment (9 progressive disease, 2 adverse event [AE], 2 investigator decision, 1 withdrawal by patient). The median age was 71 years (range, 37-84) and 67.9% were men. RR patients (49.1%) received a median of 2 lines of prior therapy (range, 1-8). The median follow-up time was 14.8 months (range, 1.4-27.3). Forty-eight (90.6%) patients experienced ≥1 treatment-emergent adverse event (TEAE). The most common any-grade AEs are summarized in Table 1. Twenty-two (41.5%) patients experienced grade ≥3 AEs; the most common grade ≥3 AEs were neutrophil count decreased (9.4%), platelet count decreased (9.4%), and neutropenia (5.7%). One patient with RR MCL experienced a fatal TEAE of septic shock. Thirty-five (66%) patients had ≥1 TEAE of special interest, including 21 (39.6%) patients with hemorrhage, 17 (32.1%) with infections, and 11 (20.8%) with thrombocytopenia. The most common grade ≥3 TEAEs of special interest were neutropenia (15.1%), infections (11.3%), hemorrhage (9.4%), and thrombocytopenia (9.4%). Investigator-assessed disease responses are reported from part 2. Among patients with MCL, the overall response rate (ORR) was 81.8%. Among patients with CLL/SLL, the ORR (partial response with lymphocytosis or better) was 92.9% in treatment-naïve (TN) patients and 100% in RR patients. The ORR among patients with WM was 92.3%, including 15.4% very good partial response (VGPR) in TN patients, and 100%, including 66.7% VGPR, in RR patients (Table 2). The median duration of overall response has not been reached for any disease cohort.
Conclusion: Preliminary data from this phase 1/2 study demonstrate that zanubrutinib can be an efficacious and safe BTK inhibitor for Japanese patients with RR MCL, CLL/SLL, and WM. Safety and efficacy data are comparable with those observed in published data across ethnic groups.
Disclosures
Shimada:CHUGAI PHARMACEUTICAL: Consultancy, Honoraria, Research Funding; Daiichi sankyo: Consultancy, Honoraria, Research Funding; Kyowa Kirin: Research Funding; Bristol-Meyers Squibb: Consultancy, Honoraria; Celgene: Honoraria, Research Funding; AbbVie: Consultancy; Novartis: Consultancy, Honoraria; Meiiji Seika: Consultancy; Celgene: Honoraria, Research Funding; Kyowa Kirrin: Research Funding; Otsuka: Research Funding; Eisai: Honoraria, Research Funding; AstraZeneca: Honoraria; Janssen: Honoraria; Takeda: Honoraria; Symbio: Honoraria; Asclepia: Honoraria. Kondo:Chugai Pharmaceutical: Honoraria; Alexion Pharma: Honoraria; PharmaEssentia Japan: Honoraria. Sakai:Chugai Pharmaceutical Co.: Research Funding; Kyowa Hakko Kirin Co.: Honoraria, Research Funding; Taiho Pharmaceutical Co: Research Funding; Astra Zeneca plc.: Honoraria; Takeda Pharmaceutical Co.: Honoraria. Sunami:AbbVie: Research Funding; BMS: Honoraria, Research Funding; Celgene: Research Funding; Chugai: Research Funding; GSK: Research Funding; Janssen: Honoraria, Research Funding; Ono: Honoraria, Research Funding; Otsuka: Research Funding; Sanofi: Honoraria, Research Funding; Takeda: Research Funding. Guo:BeiGene Ltd: Current Employment, Current equity holder in publicly-traded company, Other: Travel, Accommodations, and Expenses. Novotny:BeiGene: Current Employment, Current equity holder in publicly-traded company. Tankersley:BeiGene Ltd: Current Employment, Current equity holder in publicly-traded company, Other: Travel, Accommodations, Expenses. Takai:Varian Medical Systems: Ended employment in the past 24 months; BeiGene USA: Current Employment, Current equity holder in publicly-traded company, Other: travel, accommodations, expenses. Yao:BeiGene Ltd: Current Employment, Current equity holder in publicly-traded company, Other: travel, accommodations, expenses. Zhong:BeiGene: Current equity holder in publicly-traded company; BeiGene (Shanghai) Co. Ltd: Current Employment. Zhu:BeiGene: Current Employment, Current equity holder in publicly-traded company. Izutsu:Ono Pharmaceutical: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Janssen: Research Funding; Beigene: Consultancy, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Chugai: Consultancy, Honoraria, Research Funding; Eli Lilly and Company: Consultancy, Honoraria; Merck Sharp & Dohme: Honoraria, Research Funding; Eizai: Honoraria, Research Funding; Incyte: Research Funding; Yakult: Research Funding; Kyowa Kirin: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; Genmab: Research Funding; Loxo Oncology: Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Symbio: Honoraria; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.